medical packaging
Medical packaging play a vital role in process of manufacturing medical devices – safety of conducting proper medical procedure depends on their performance quality. Medical packaging are subjected to various sterilization processes, and therefore must be designed in a way that allows the penetration of sterilizing factor into the package, which contains the components in accordance with a particular medical procedure.
medical printing house - flexographic printing in a clean room standard
We are one of the leading manufacturers of advanced medical packaging acting as a sterile barrier for medical devices. We offer flexographic printing services dedicated to the healthcare industry, meeting the highest quality and product safety standards. Our offer is directed both to manufacturers of sterilized products and to professionals providing services in the healthcare industry. We offer printing on medical paper, lamianates, non-woven fabric and Tyvek® 1059B and 2FS. We offer multicolour printing using water-based, specialised flexographic inks for sterilisation by ethylene oxide (EO), steam (STEAM), formaldehyde (FORM), plasma (VH2O2).
more

medical printing house
We offer multicolour printing using water-based, specialised flexographic inks for sterilisation by ethylene oxide (EO), steam (STEAM), formaldehyde (FORM), plasma (VH2O2). Prints are made in a production hall that meets the ISO 9 clean room standard. The process of printing is supported by the process of materials packing, i.e.: rewinding, cutting, packing. Strictly controlled production conditions are ensured by a HEPA filtration system, clean room airlock and the adequate pressure levels in the various production rooms. They guarantee safe conditions for the production of packaging and semi-finished products for the healthcare market.
As part of our cooperation, we offer wide- and narrow-web flexo printing that meets the requirements of the "Food Packaging" directive, certified raw materials from world-class suppliers, graphic preparation for printing as well as technical advice and fast turnaround times.

BOM - safe medical packaging
BOM the safe medical packaging are dedicated for wrapping medical devices, intended for sterilization by steam, ethylene oxide, irradiation and ionized gas based on hydrogen peroxide plasma. Prior to sterilization all products have to be wrapped in specially designed packages. If used correctly, BOM medical packaging ensures sterility of the item inside within a given period of time. The pouches and reels are made of 2 different types of raw material –transparent film with barrier properties and the material that enables free access of the sterilizing agent to the item inside and in the form of medical paper or other special material suitable for such usage e.g. Tyvek® with high parameters of microbiological barrier.
more
BOM - safe medical packaging
On packaging the are chemical sterilization indicators which change color due to sterilizing factor and inform the user about the method of sterilization that was used and allow to distinguish the medical sets before sterilization and after. The presence of three indicators on one package allows the safe use for different sterilizing methods and minimizes the costs associated with additional stock.
The sterilization packaging are specially made to protect the sterile material inside from contamination i.e.: dressings, surgical instruments, op-drapes, surgical clothing and other materials dedicated for direct contact with patient's body especially with open wound and body fluids.
BOM packaging are produced in controlled environmental conditions in terms of microbiological aspects and cleanliness particulate matters - clean room. The production environment complies with the exacting standard of EN ISO 14644-1.
unique and complex solution
Our goal is to provide a comprehensive offer for the client, taking into account safety and responsibility for the quality of products and services provided within the medical business. We are the only company in Poland that offers comprehensive solutions, bearing in mind production of medical products, packing them in a safe medical packaging and sterilization using ethylene oxide or steam.
rigid blister – complex solution
We are in favour of more and more advanced technical solutions and high quality demands in the medical industry, that is why Plastica Ltd. invested in machines to produce medical devices packed in soft and rigid blister in accordance with the ISO 11607 norm. The technical potential of this machine is immense; we offer medical packaging of a 450x525mm size with any internal divisions that depend on the content. The unquestionable advantage of our packaging is the possibility of using the same blister as a liquid container, it increases the rigidity and strength of the packaging as well as the better organization of elements during the usage.
more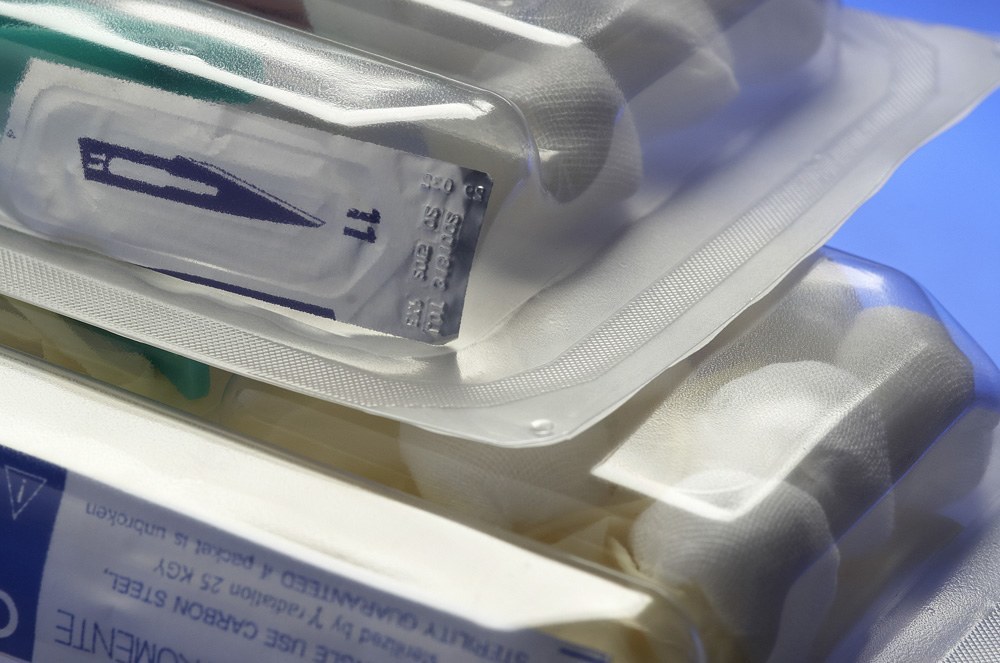

rigid blister – complex solution
The soft and rigid packaging is thermoformed into the depth of 70mm and 40 mm adequately. Production of medical packaging and medical devices is done in a clean area - CLEAN ROOM.
The barrier type of foil packaging is tightly closed with medical paper, through which the ethylene oxide pervades during the sterilization process. The rigid and soft blister packaging is characterized by a high quality weld, in accordance with the PN-EN 868-5:2009 norm. The medical devices production is conducted in accordance with the ISO 14644-1:2005 union norm in class 8.
Our company offers also the service of packing medical devices together with the ethylene oxide sterilization process.
Polimery
Plastica Sp. z o.o. wspiera akcję społeczną POLIMERY PRZYJAZNE CZŁOWIEKOWI, POTRZEBNE LUDZIOM
Niewiele jest spraw, co do których Polacy są zgodni. Jedną z nich jest fatalny publiczny wizerunek tworzyw sztucznych. Przemysł tworzyw stał się dyżurnym „chłopcem do bicia” w Polsce i UE, jest obwiniany za wszelkie błędy i wypaczenia naszej cywilizacji. Czas na podjęcie działań, które w rozsądnej perspektywie pozwolą zmienić negatywne społeczne nastawienie wobec tworzyw.
Przede wszystkim najwyższy czas na wyrugowanie z przestrzeni publicznej koszmarka językowego „tworzywa sztuczne”. Ten termin natychmiast odrzuca każdego normalnego człowieka, jego ocieplenie i zmiana wizerunku na pozytywny wydaje się niemożliwa, albowiem nic co „sztuczne” nie może być przyjazne człowiekowi. Dlatego proponujemy zastąpienie koszmarka językowego terminem „tworzywa polimerowe”, a szerzej POLIMERY. Nazwa POLIMERY oddaje istotę rzeczy i nie jest obciążona negatywnymi asocjacjami i skojarzeniami wytworzonymi wokół koszmarka językowego. Jest nawet sympatyczna i, jak widać po logo akcji, da się za jej pomocą komunikować treści mające odzwierciedlenie w rzeczywistości.
Pamiętajmy, że jak mówi Pismo, na początku było Słowo. Język ma kluczowe znaczeniu w międzyludzkiej komunikacji i tworzeniu korzystnego lub negatywnego wizerunku. Krótko mówiąc: język tworzy rzeczywistość.
Celem akcji jest rozpropagowanie terminu POLIMERY, który w niedalekiej przyszłości powinien zastąpić koszmarek językowy. Przemysł polimerowy musi się zacząć w końcu bronić. Polimery są niezbywalnym elementem naszej cywilizacji i tylko od człowieka, od nas samych zależy, jak będą nam służyć.
Wprowadzenie terminu POLIMERY do dyskursu publicznego pozwoli rozpocząć wiele pozytywnych działań wizerunkowych we współpracy z mediami i organizacjami społecznymi.
Zmiana wizerunku branży powinna ożywczo wpłynąć na sam biznes. Zejście z linii strzału ekologów, mediów, polityków, innych grup opiniotwórczych, powinno przynieść mierzalne biznesowe efekty. Taki stan rzeczy na pewno zostanie zauważony przez inwestorów zagranicznych. Wreszcie, może to być sygnał do konsolidacji przemysłu polimerowego, co zaowocuje wzrostem siły i znaczenia tej branży w całej gospodarce.






